
Medical Technology Jobs Skills Radar 2026: Emerging Tools, Devices & Digital Platforms to Learn Now
From AI-powered diagnostics to wearable health monitors and smart surgical robotics, the UK’s medical technology sector is entering a new era. As the NHS and private health providers continue to embrace innovation, demand for talent is rising rapidly across engineering, digital health, regulatory affairs, and data-driven medical product development.
Welcome to the Medical Technology Jobs Skills Radar 2026—your annual guide to the tools, platforms, techniques and regulatory skills shaping the UK’s medical technology jobs market. Whether you're a biomedical engineer, regulatory specialist, or medtech software developer, this radar helps you align your skills with future hiring demand.
Why Medtech Skills Are Evolving in 2026
The UK’s Life Sciences Vision and NHSX-backed innovation push are driving major change in medtech hiring. Employers increasingly expect candidates who can:
Design and validate medical devices
Work across embedded, mobile, and cloud-connected systems
Understand regulatory and risk frameworks (MHRA, UKCA, ISO 13485)
Collaborate in multi-disciplinary product teams
Combine software, electronics, AI, and data science fluency
Clinical safety, human-centred design and real-world performance are now part of every role—from firmware to product management.
Top Engineering & Technical Skills in 2026
1. Medical Device Design (CAD + Simulation)
What it is: 3D design and stress simulation for medical tools, implants, prosthetics and components.
Why it matters: Forms the backbone of medtech hardware development.
Used by: Smith & Nephew, Medtronic UK, NHS innovation units.
Roles: Biomedical Design Engineer, R&D Engineer, Mechanical Medtech Designer.
Tools to pair: SolidWorks, Autodesk Fusion 360, COMSOL, ANSYS.
2. Embedded Systems & Firmware
What it is: Microcontroller programming and sensor integration for medical hardware.
Why it matters: Powers infusion pumps, monitors, wearable trackers, diagnostic devices.
Used by: Medtech startups, ventilator developers, wearable manufacturers.
Languages: C/C++, MicroPython.
Roles: Embedded Firmware Engineer, IoT Medical Device Developer.
3. Signal Processing for Biosensors
What it is: Processing ECG, EEG, EMG and other biometric signals from analog sources.
Why it matters: Enables smart monitoring and predictive diagnostics.
Used by: Health wearable firms, neurotech labs, sports tech.
Tools to pair: MATLAB, LabVIEW, Python (SciPy), digital filter design.
4. Medical Imaging & Image Analysis
What it is: Software tools for CT/MRI/DICOM data processing and AI diagnostics.
Why it matters: Rapid growth in radiology AI and 3D visualisation tools.
Used by: GE Healthcare, NHS Trusts, AI radiology startups.
Tools: DICOM viewer software, 3D Slicer, OpenCV, ITK.
5. Additive Manufacturing (3D Printing for Medtech)
What it is: Used to prototype and produce dental implants, hearing aids, orthopaedic devices.
Why it matters: Allows personalisation, rapid iteration, and novel geometries.
Used by: Dental labs, orthopaedic innovators, surgical teams.
Tools: Formlabs PreForm, Autodesk Netfabb, Ultimaker Cura.
In-Demand Software & Data Tools for Medtech
1. Python & R for Medical Data
Why it matters: Used for data preprocessing, predictive modelling, clinical outcome analysis.
Used by: Clinical AI startups, pharma analytics, diagnostic tool developers.
Libraries to learn: pandas, scikit-learn, NumPy, statsmodels, caret (R), ggplot2.
2. MATLAB / Simulink
Why it matters: Still standard for physiological modelling, signal simulation, and embedded medical systems.
Used by: Medical hardware manufacturers, device modelling teams.
3. FHIR / HL7 / DICOM
What it is: Data formats and protocols used to communicate with hospital systems and EHRs.
Why it matters: Interoperability is essential in regulated healthcare.
Used by: Digital health apps, NHS integrations, radiology tools.
Roles: Health IT Developer, Medtech Data Integrator.
4. Mobile Health App Development
Why it matters: Regulated mobile apps are growing fast in chronic care and monitoring.
Used by: Ada Health, Kaia Health, UK medical app developers.
Skills to learn: Flutter, React Native, Swift, Bluetooth LE integration.
5. Cloud & IoT Platforms
Why it matters: Used for fleet monitoring of devices, remote diagnostics, AI model deployment.
Tools to learn: AWS IoT Core, Azure IoT Hub, Google Cloud Healthcare API.
AI & Machine Learning in Medtech
▸ scikit-learn / TensorFlow / PyTorch
Use cases: Diagnostic tools, ECG analysis, risk stratification.
Roles: Clinical AI Scientist, ML Engineer in Health.
▸ Computer Vision in Imaging
Tools: OpenCV, Keras, YOLOv5 for diagnostics or surgical vision systems.
Used by: ScanNav, Skin Analytics, and NHS AI award-winning projects.
▸ Explainable AI (XAI)
Tools: SHAP, LIME, Fairlearn.
Why it matters: Clinical decisions must be transparent, especially under MHRA guidelines.
Compliance, QA & Regulatory Knowledge
1. ISO 13485 & ISO 14971
What it is: Standards for medical device quality and risk management.
Why it matters: Required by almost every UK medtech employer.
Roles: QA/RA Associate, Compliance Manager, Clinical Evaluator.
2. UKCA / CE / MDR Compliance
What it is: Required for placing medical devices on the UK and EU market.
Skills to learn: Technical documentation, GSPR checklist, notified body submissions.
3. SaMD (Software as a Medical Device)
Why it matters: Many modern products are digital-first. Even mobile apps may require CE marking.
Used by: AI health startups, wellness apps entering regulated markets.
4. GAMP 5 & Validation
What it is: Guidance for validating computerised systems in medical environments.
Used by: Pharma, diagnostics, NHS IT.
5. LIMS & QMS Platforms
What it is: Systems for managing samples, tracking lab work, and supporting regulatory compliance.
Used by: Testing labs, pharma R&D, diagnostics.
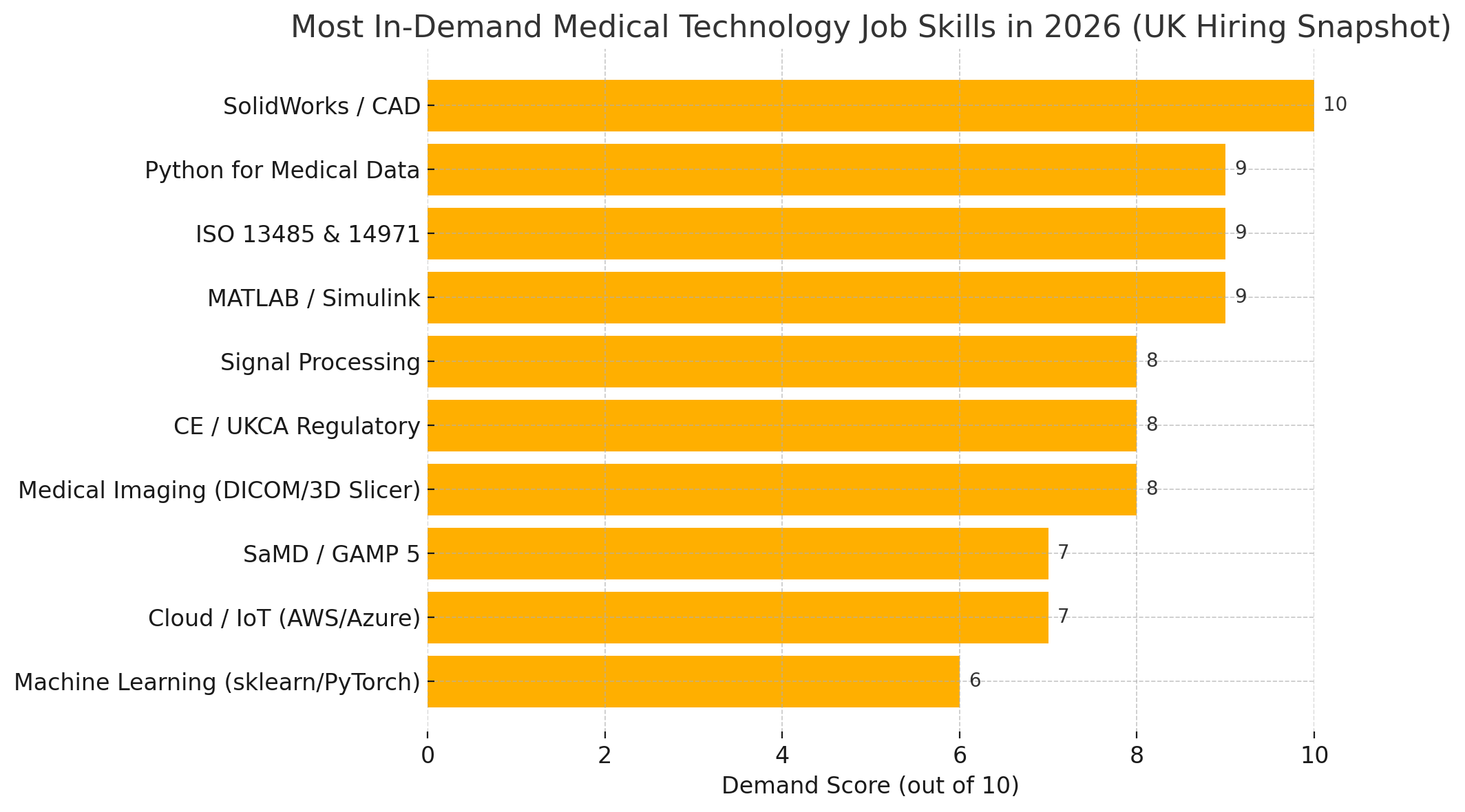
Most In-Demand Medical Technology Job Skills in 2026 (UK Hiring Snapshot Forecast)
Let’s visualise the skills, platforms & standards most in demand across the UK medtech sector:
How to Future-Proof Your Medical Technology Career in 2026
Bridge Engineering with RegulationUK employers love medtech candidates who understand both device design and compliance.
Learn Python + MATLABCombining these gives you both data science power and control system fluency.
Understand SaMD, AI & Cloud RequirementsSoftware and connected devices are now core to the NHS & international markets.
Build a Multi-Disciplinary ProfileFrom hardware and embedded systems to clinical workflow understanding—breadth helps.
Engage with UK Medtech EcosystemsJoin ABHI, attend Med-Tech Innovation Expo, and engage with NIHR and MHRA-led projects.
Where to Find Medical Technology Jobs in the UK
🩺 Browse the latest verified listings at www.medicaltechnologyjobs.co.uk—from graduate biomedical engineer roles to digital therapeutics, QA/RA, and medtech data science jobs.
Conclusion: Your Medtech Career Toolkit for 2026
The UK medical technology sector is booming—but roles are becoming more complex. Those who combine technical know-how with digital tools and regulatory literacy will lead the next wave of medtech innovation.
Use this Medical Technology Jobs Skills Radar 2026 to shape your skills, training and job search. We update it annually to reflect real demand from NHS suppliers, global medtech firms, and innovative startups across the UK.
Subscribe to our newsletter for jobs, salary guides, tech updates & career insights.


